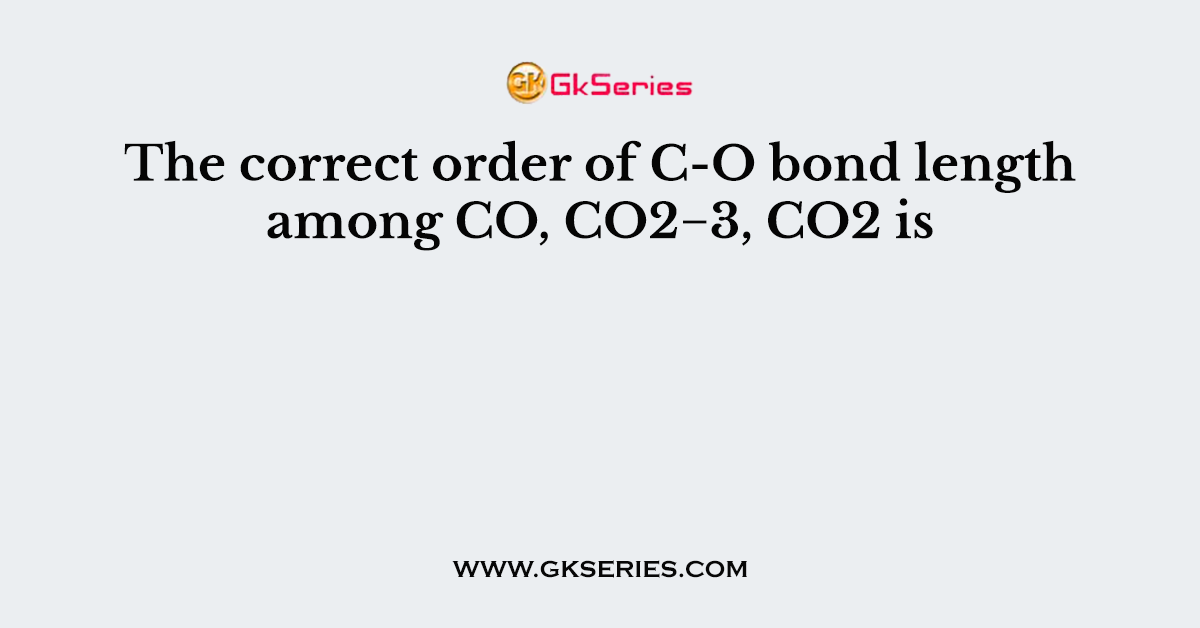
Q. The correct order of C-O bond length among CO, CO2−3, CO2 is
- CO2 < CO2−3< CO
- CO = CO2 < CO2−3
- CO2−3< CO2 < CO
- CO < CO2 < CO2−3
Answer: CO < CO2 < CO2−3

Answer: CO < CO2 < CO2−3
| Government Schemes Mock Test | Start Test! |
| Political Science Mock Test – 42 | Start Test |
| History Test – 190 | Start Test |
| Quantitative Aptitude Test | Start Test! | Data Interpretation - Mock Test | Start Test! |
| General Awareness - Mock Test | Start Test! |
| Reasoning Ability - Mock Test | Start Test! |