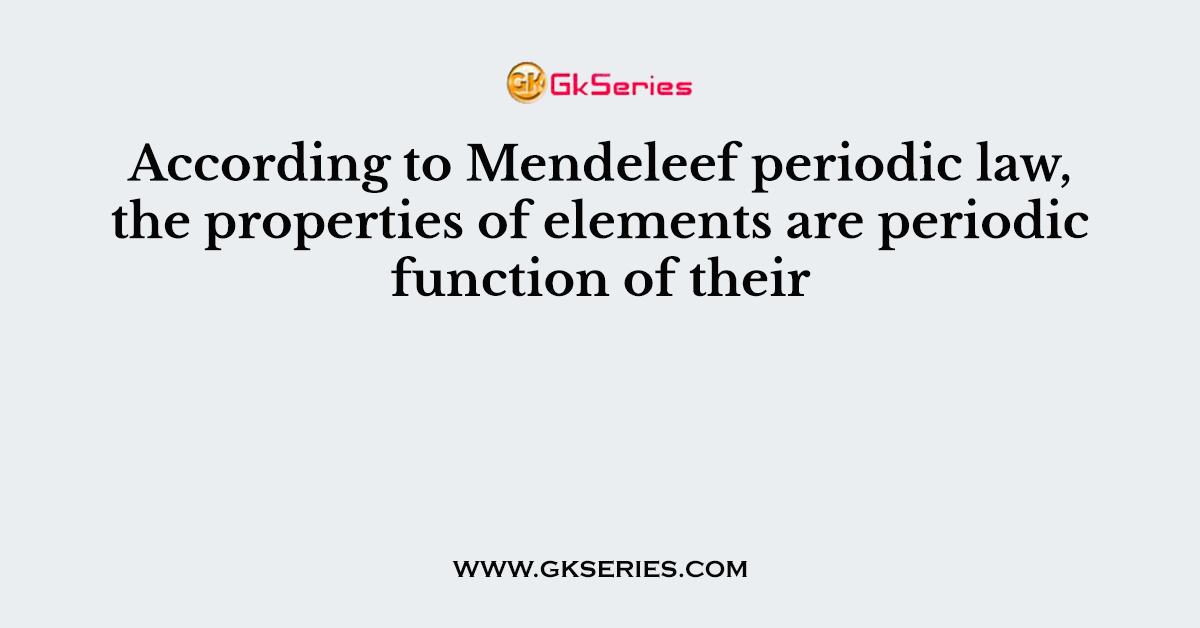
Q. Which of the following statements is incorrect?
A. I.E.1 of O is lower than that of N but I.E.2 O is higher than that of N
B. The enthalpy of N to gain an electron is almost zero but of P is 74.3 kJ mol-1
C. isoelectronic ions belong to the same period
D. The covalent radius of iodine is less than its Van der Waal’s radius
Answer: isoelectronic ions belong to the same period