There are six groups of p–block elements in the periodic table numbering from 13 to 18. Boron, carbon, nitrogen, oxygen, fluorine and helium head the groups.
Group 15 Elements
Group 15 includes nitrogen, phosphorus, arsenic, antimony, bismuth and moscovium.
Occurrence: Molecular nitrogen comprises 78% by volume of the atmosphere. In the earth’s crust, it occurs as sodium nitrate, NaNO3(called Chile saltpetre) and potassium nitrate (Indian saltpetre). It is found in the form of proteins in plants and animals. Phosphorus occurs in minerals of the apatite family which are the main components of phosphate rocks. Phosphorus is an essential constituent of animal and plant matter. It is present in bones as well as in living cells. Phosphoproteins are present in milk and eggs. Arsenic, antimony and bismuth are found mainly as sulphide minerals. Moscovium is a synthetic radioactive element.
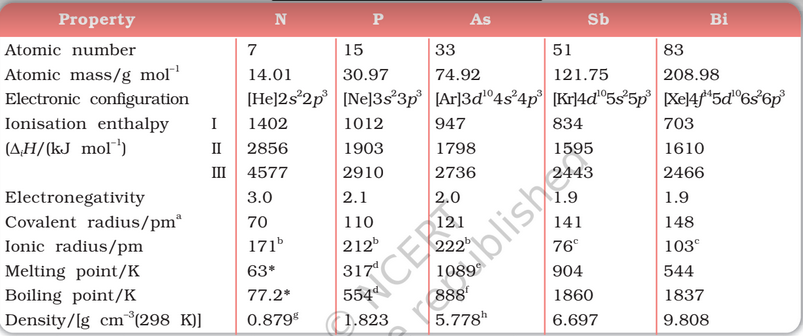
Electronic Configuration: The valence shell electronic configuration of these elements is ns2np3. The s orbital in these elements is completely filled and p orbitals are half-filled, making their electronic configuration extra stable.
Atomic and Ionic Radii: Covalent and ionic (in a particular state) radii increase in size down the group. There is a considerable increase in covalent radius from N to P. However, from As to Bi only a small increase in covalent radius is observed. This is due to the presence of completely filled d and/or f orbitals in heavier members.
Ionisation Enthalpy: Ionisation enthalpy decreases down the group due to gradual increase in atomic size. Because of the extra stable half-filled p orbitals electronic configuration and smaller size, the ionisation enthalpy of the group 15 elements is much greater than that of group 14 elements in the corresponding periods.
Electronegativity: The electronegativity value, in general, decreases down the group with increasing atomic size. However, amongst the heavier elements, the difference is not that much pronounced.
Physical Properties: All the elements of this group are polyatomic. Dinitrogen is a diatomic gas while all others are solids. Metallic character increases down the group. Nitrogen and phosphorus are non-metals, arsenic and antimony metalloids and bismuth is a metal. This is due to decrease in ionisation enthalpy and increase in atomic size. The boiling points, in general, increase from top to bottom in the group but the melting point increases upto arsenic and then decreases upto bismuth. Except nitrogen, all the elements show allotropy.
Chemical Properties:
- Oxidation states and trends in chemical reactivity: The common oxidation states of these elements are –3, +3 and +5. The tendency to exhibit –3 oxidation state decreases down the group due to increase in size and metallic character.
- Anomalous properties of nitrogen: Nitrogen differs from the rest of the members of this group due to its small size, high electronegativity, high ionisation enthalpy and non-availability of d orbitals.
Dinitrogen
Preparation: Dinitrogen is produced commercially by the liquefaction and fractional distillation of air. Liquid dinitrogen (b.p. 77.2 K) distils out first leaving behind liquid oxygen (b.p. 90 K). In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
![]()
Properties: Dinitrogen is a colourless, odourless, tasteless and non-toxic gas. Nitrogen atom has two stable isotopes: 14N and 15N. It has a very low solubility in water (23.2 cm3 per litre of water at 273 K and 1 bar pressure) and low freezing and boiling points.
Uses: The main use of dinitrogen is in the manufacture of ammonia and other industrial chemicals containing nitrogen, (e.g., calcium cyanamide). It also finds use where an inert atmosphere is required (e.g., in iron and steel industry, inert diluent for reactive chemicals). Liquid dinitrogen is used as a refrigerant to preserve biological materials, food items and in cryosurgery.
Ammonia
Preparation: Ammonia is present in small quantities in air and soil where it is formed by the decay of nitrogenous organic matter e.g., urea.
![]()
Properties: Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass.
Uses: Ammonia is used to produce various nitrogenous fertilisers (ammonium nitrate, urea, ammonium phosphate and ammonium sulphate) and in the manufacture of some inorganic nitrogen compounds, the most important one being nitric acid. Liquid ammonia is also used as a refrigerant.
Oxides of Nitrogen
Nitrogen forms a number of oxides in different oxidation states. Two of the most toxicologically significant compounds are nitric oxide (NO) and nitrogen dioxide (NO2). Other gases belonging to this group are nitrogen monoxide (or nitrous oxide, N2O), and nitrogen pentoxide (NO5).
NO2 contains odd number of valence electrons. It behaves as a typical odd molecule. On dimerisation, it is converted to stable N2O4 molecule with even number of electrons.
Nitric Acid
Nitrogen forms oxoacids such as H2N2O2 (hyponitrous acid), HNO2 (nitrous acid) and HNO3 (nitric acid). Amongst them HNO3 is the most important.
Preparation: In the laboratory, nitric acid is prepared by heating KNO3 or NaNO3 and concentrated H2SO4 in a glass retort.
![]()
Properties: It is a colourless liquid (f.p. 231.4 K and b.p. 355.6 K). Laboratory grade nitric acid contains ~ 68% of the HNO3 by mass and has a specific gravity of 1.504.
Uses: The major use of nitric acid is in the manufacture of ammonium nitrate for fertilisers and other nitrates for use in explosives and pyrotechnics. It is also used for the preparation of nitroglycerin, trinitrotoluene and other organic nitro compounds. Other major uses are in the pickling of stainless steel, etching of metals and as an oxidiser in rocket fuels.
Phosphorus: Allotropic Forms
Phosphorus is found in many allotropic forms, the important ones being white, red and black.
White phosphorus is a translucent white waxy solid. It is poisonous, insoluble in water but soluble in carbon disulphide and glows in dark (chemiluminescence). It dissolves in boiling NaOH solution in an inert atmosphere giving PH3.
![]()
Red phosphorus is obtained by heating white phosphorus at 573K in an inert atmosphere for several days. When red phosphorus is heated under high pressure, a series of phases of black phosphorus is formed.
Black phosphorus has two forms α-black phosphorus and β-black phosphorus. α-Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803K.
Phosphine
Preparation: Phosphine is prepared by the reaction of calcium phosphide with water or dilute HCl.
Properties: It is a colourless gas with rotten fish smell and is highly poisonous. It explodes in contact with traces of oxidising agents like HNO3, Cl2 and Br2 vapours.
Uses: The spontaneous combustion of phosphine is technically used in Holme’s signals. Containers containing calcium carbide and calcium phosphide are pierced and thrown in the sea when the gases evolved burn and serve as a signal. It is also used in smoke screens.
Phosphorus Halides:
Phosphorus forms two types of halides, PX3 (X = F, Cl, Br, I) and PX5 (X = F, Cl, Br).
Phosphorus Trichloride
Preparation: It is obtained by passing dry chlorine over heated white phosphorus.
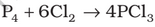
It is also obtained by the action of thionyl chloride with white phosphorus.
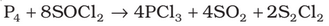
Properties: It is a colourless oily liquid and hydrolyses in the presence of moisture.
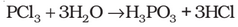
Phosphorus Pentachloride
It has a pyramidal shape as shown, in which phosphorus is sp3 hybridised.
Preparation: Phosphorus pentachloride is prepared by the reaction of white phosphorus with excess of dry chlorine
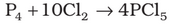
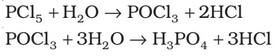
Oxoacids of Phosphorus
Oxoacids are basically acids that contain the element oxygen. As such, Phosphorus is known to form a number of oxoacids, for example: H3PO4, H3PO3, etc. In oxoacids of phosphorus, it is tetrahedrally surrounded by other atoms. Generally, all these acids are known to form at least one P=O bond and one P–OH bond.
Phosphorus acid, H3PO3: Phosphorous acid is a diprotic acid that is, it ionizes two protons. It is better described with the structural formula HPO(OH)2 Phosphorous acid is prepared by hydrolysis of phosphorus trichloride with acid or steam.
Phosphoric acid, H3PO4: Phosphoric acid is a triprotic acid that is, it ionizes three protons. It is a non-toxic acid, when pure and is a solid at room temperature and pressure. Phosphoric acid is prepared by adding sulphuric acid to tricalcium phosphate rock:
Group 16 Elements
Oxygen, sulphur, selenium, tellurium, polonium and livermorium constitute Group 16 of the periodic table. This is sometimes known as group of chalcogens.
Occurrence: Oxygen is the most abundant of all the elements on earth. Oxygen forms about 46.6% by mass of earth’s crust. Dry air contains 20.946% oxygen by volume.
Electronic Configuration: The elements of Group16 have six electrons in the outermost shell and have ns2 np4 general electronic configuration.
Atomic and Ionic Radii: Due to increase in the number of shells, atomic and ionic radii increase from top to bottom in the group. The size of oxygen atom is, however, exceptionally small.
Ionisation Enthalpy: Ionisation enthalpy decreases down the group. It is due to increase in size. However, the elements of this group have lower ionisation enthalpy values compared to those of Group15 in the corresponding periods. This is due to the fact that Group 15 elements have extra stable half-filled p orbitals electronic configurations.
Electron Gain Enthalpy: Because of the compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur. However, from sulphur onwards the value again becomes less negative upto polonium.
Electronegativity: Next to fluorine, oxygen has the highest electronegativity value amongst the elements. Within the group, electronegativity decreases with an increase in atomic number. This implies that the metallic character increases from oxygen to polonium.
Physical Properties: Oxygen and sulphur are non-metals, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived (Half-life 13.8 days). All these elements exhibit allotropy. The melting and boiling points increase with an increase in atomic number down the group.
Chemical Properties: The elements of this group react with hydrogen to form hydrides of the type H2E, where E could be oxygen, sulphur, selenium, tellurium or polonium.

Dioxygen
Preparation: Dioxygen can be obtained in the laboratory by the following ways:
(i) By heating oxygen containing salts such as chlorates, nitrates and permanganates.
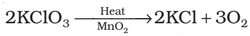
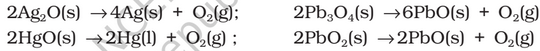
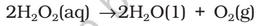
Properties: Dioxygen is a colourless and odourless gas. Its solubility in water is to the extent of 3.08 cm3 in 100 cm3 water at 293 K which is just sufficient for the vital support of marine and aquatic life.
Uses: In addition to its importance in normal respiration and combustion processes, oxygen is used in oxyacetylene welding, in the manufacture of many metals, particularly steel. Oxygen cylinders are widely used in hospitals, high altitude flying and in mountaineering. The combustion of fuels, e.g., hydrazines in liquid oxygen, provides tremendous thrust in rockets.
Simple Oxides
Oxides can be simple (e.g., MgO, Al2O3) or mixed (Pb3O4, Fe3O4). Simple oxides can be classified on the basis of their acidic, basic or amphoteric character. An oxide that combines with water to give an acid is termed acidic oxide (e.g., SO2, Cl2O7, CO2, N2O5). For example, SO2 combines with water to give H2SO3, an acid.
Ozone
Ozone is an allotropic form of oxygen. It is too reactive to remain for long in the atmosphere at sea level. At a height of about 20 kilometres, it is formed from atmospheric oxygen in the presence of sunlight. This ozone layer protects the earth’s surface from an excessive concentration of ultraviolet (UV) radiations.
Preparation: When a slow dry stream of oxygen is passed through a silent electrical discharge, conversion of oxygen to ozone (10%) occurs. The product is known as ozonised oxygen.
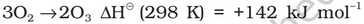
Properties: Pure ozone is a pale blue gas, dark blue liquid and violet-black solid. Ozone has a characteristic smell and in small concentrations it is harmless. However, if the concentration rises above about 100 parts per million, breathing becomes uncomfortable resulting in headache and nausea.
Uses: It is used as a germicide, disinfectant and for sterilising water. It is also used for bleaching oils, ivory, flour, starch, etc. It acts as an oxidising agent in the manufacture of potassium permanganate.
Sulphur: Allotropic Forms
Sulphur forms numerous allotropes of which the yellow rhombic (α-sulphur) and monoclinic (β -sulphur) forms are the most important. The stable form at room temperature is rhombic sulphur, which transforms to monoclinic sulphur when heated above 369 K.
Rhombic sulphur (α-sulphur): This allotrope is yellow in colour, m.p. 385.8 K and specific gravity 2.06. Rhombic sulphur crystals are formed on evaporating the solution of roll sulphur in CS2. It is insoluble in water but dissolves to some extent in benzene, alcohol and ether. It is readily soluble in CS2.
Monoclinic sulphur (β-sulphur): Its m.p. is 393 K and specific gravity 1.98. It is soluble in CS2. This form of sulphur is prepared by melting rhombic sulphur in a dish and cooling, till crust is formed.
Sulphur Dioxide
Preparation: Sulphur dioxide is formed together with a little (6-8%) sulphur trioxide when sulphur is burnt in air or oxygen
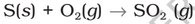
Properties: Sulphur dioxide is a colourless gas with pungent smell and is highly soluble in water. It liquefies at room temperature under a pressure of two atmospheres and boils at 263 K.
Uses: Sulphur dioxide is used (i) in refining petroleum and sugar (ii) in bleaching wool and silk and (iii) as an anti-chlor, disinfectant and preservative. Sulphuric acid, sodium hydrogen sulphite and calcium hydrogen sulphite (industrial chemicals) are manufactured from sulphur dioxide. Liquid SO2 is used as a solvent to dissolve a number of organic and inorganic chemicals.
Oxoacids of Sulphur
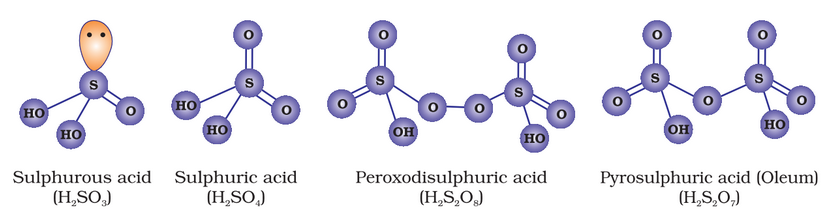
Sulphuric Acid
Manufacture: Sulphuric acid is one of the most important industrial chemicals worldwide. Sulphuric acid is manufactured by the Contact Process which involves three steps:
(i) burning of sulphur or sulphide ores in air to generate SO2.
(ii) conversion of SO2 to SO3 by the reaction with oxygen in the presence of a catalyst (V2O5), and
(iii) absorption of SO3 in H2SO4 to give Oleum (H2S2O7).
Properties: Sulphuric acid is a colourless, dense, oily liquid with a specific gravity of 1.84 at 298 K. The acid freezes at 283 K and boils at 611 K. It dissolves in water with the evolution of a large quantity of heat. Hence, care must be taken while preparing sulphuric acid solution from concentrated sulphuric acid.
Uses: Sulphuric acid is a very important industrial chemical. A nation’s industrial strength can be judged by the quantity of sulphuric acid it produces and consumes. It is needed for the manufacture of hundreds of other compounds and also in many industrial processes. The bulk of sulphuric acid produced is used in the manufacture of fertilisers (e.g., ammonium sulphate, superphosphate). Other uses are in: (a) petroleum refining (b) manufacture of pigments, paints and dyestuff intermediates (c) detergent industry (d) metallurgical applications (e.g., cleansing metals before enameling, electroplating and galvanising (e) storage batteries (f) in the manufacture of nitrocellulose products and (g) as a laboratory reagent.
Group 17 Elements
Fluorine, chlorine, bromine, iodine, astatine and tennessine are members of Group 17. These are collectively known as the halogens. The halogens are highly reactive non-metallic elements.
Occurrence: Fluorine and chlorine are fairly abundant while bromine and iodine less so. Fluorine is present mainly as insoluble fluorides (fluorspar CaF2, cryolite Na3AlF6 and fluoroapatite 3Ca3 (PO4)2 .CaF2) and small quantities are present in soil, river water plants and bones and teeth of animals. Sea water contains chlorides, bromides and iodides of sodium, potassium, magnesium and calcium, but is mainly sodium chloride solution (2.5% by mass).
Electronic Configuration: All these elements have seven electrons in their outermost shell (ns2np5) which is one electron short of the next noble gas.
Atomic and Ionic Radii: The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge. The atomic radius of fluorine like the other elements of second period is extremely small. Atomic and ionic radii increase from fluorine to iodine due to increasing number of quantum shells.
Ionisation Enthalpy: They have little tendency to lose electron. Thus they have very high ionisation enthalpy. Due to increase in atomic size, ionisation enthalpy decreases down the group.
Electron Gain Enthalpy: Halogens have maximum negative electron gain enthalpy in the corresponding periods. This is due to the fact that the atoms of these elements have only one electron less than stable noble gas configurations.
Electronegativity: They have very high electronegativity. The electronegativity decreases down the group. Fluorine is the most electronegative element in the periodic table.
Physical Properties: Halogens display smooth variations in their physical properties. Fluorine and chlorine are gases, bromine is a liquid and iodine is a solid. Their melting and boiling points steadily increase with atomic number. All halogens are coloured.
Chemical Properties:
A. Oxidizing Power: Halogens are great oxidizing agents. Fluorine can oxidize all halide particles to halogen in a solution. However, oxidizing power decreases as we move down the group.
Chlorine can oxidize bromide to bromine and iodide to iodine.
Cl₂ + 2Br¯ → Br₂ + 2Cl¯
Cl₂ + 2I¯ → I₂ + 2Cl¯
Bromine can oxidize iodide to iodine.
Br₂ + 2I¯ → I + 2Br¯
B. Reaction with Hydrogen: Acidic hydrogen halides are formed when halides react with hydrogen. The reactivity of halogen towards halogen decreases as we move down group 17. Therefore, their acidity also decreases as we move down the group.
In dark
H₂ + F₂→ 2HF
In sunlight
H₂ + Cl₂ → 2HCl
Δ
H + Br₂ → 2HBr
Δ
H₂ + I₂ → 2HI
C. Reaction with Oxygen: Halogen combines with oxygen to form halogen oxides, but they are not steady. The general formula for oxides is X₂O to X₂O₇.
D. Reaction with Metals: Halogens react with metals instantly due to their high reactivity to form metal halides.
Sodium reacts with chlorine to form sodium chloride which releases a large amount of heat energy and yellow light as it is an exothermic reaction.
2Na(s) + Cl₂ (g) → 2NaCl(s)
Metal halides are ionic in nature due to the high electronegativity of halogen and electro positivity of metals. The ionic character decreases down the group.
E. Reaction with Other Halogens: Halogens form interhalogens when the react with other halogens. The general formula of interhalogens is XYn, where n = 1, 3, 5 or 7. Here X is the less electronegative halogen and Y is the more electronegative halogen.
Chlorine
Chlorine was discovered in 1774 by Scheele by the action of HCl on MnO2. In 1810 Davy established its elementary nature and suggested the name chlorine on account of its colour (Greek, chloros = greenish yellow).
Preparation: It can be prepared by any one of the following methods:
(i) By heating manganese dioxide with concentrated hydrochloric acid.
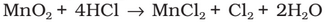
(ii) By the action of HCl on potassium permanganate.
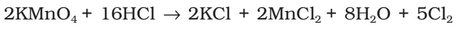
Manufacture of chlorine:
(i) Deacon’s process: By oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
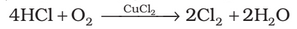
(ii) Electrolytic process: Chlorine is obtained by the electrolysis of brine (concentrated NaCl solution). Chlorine is liberated at anode. It is also obtained as a by–product in many chemical industries.
Properties: It is a greenish yellow gas with pungent and suffocating odour. It is about 2-5 times heavier than air. It can be liquefied easily into greenish yellow liquid which boils at 239 K. It is soluble in water.
Uses: It is used (i) for bleaching woodpulp (required for the manufacture of paper and rayon), bleaching cotton and textiles, (ii) in the extraction of gold and platinum (iii) in the manufacture of dyes, drugs and organic compounds such as CCl4, CHCl3, DDT, refrigerants, etc. (iv) in sterilising drinking water and (v) preparation of poisonous gases such as phosgene (COCl2), tear gas (CCl3NO2), mustard gas (ClCH2CH2SCH2CH2Cl).
Hydrogen Chloride
Glauber prepared this acid in 1648 by heating common salt with concentrated sulphuric acid. Davy in 1810 showed that it is a compound of hydrogen and chlorine.
Preparation: In laboratory, it is prepared by heating sodium chloride with concentrated sulphuric acid.
Properties: It is a colourless and pungent smelling gas. It is easily liquefied to a colourless liquid (b.p.189 K) and freezes to a white crystalline solid (f.p. 159 K).
Uses: It is used (i) in the manufacture of chlorine, NH4Cl and glucose (from corn starch), (ii) for extracting glue from bones and purifying bone black, (iii) in medicine and as a laboratory reagent.
Oxoacids of Halogens
Due to high electronegativity and small size, fluorine forms only one oxoacid, HOF known as fluoric (I) acid or hypofluorous acid. The other halogens form several oxoacids. Most of them cannot be isolated in pure state. They are stable only in aqueous solutions or in the form of their salts.
Interhalogen Compounds
When two different halogens react with each other, interhalogen compounds are formed. They can be assigned general compositions as XX′, XX3′, XX5′ and XX7′ where X is halogen of larger size and X′ of smaller size and X is more electropositive than X′.
Preparation: The interhalogen compounds can be prepared by the direct combination or by the action of halogen on lower interhalogen compounds.
Properties: These are all covalent molecules and are diamagnetic in nature. They are volatile solids or liquids at 298 K except ClF which is a gas. Their physical properties are intermediate between those of constituent halogens except that their m.p. and b.p. are a little higher than expected.
Uses: These compounds can be used as non aqueous solvents. Interhalogen compounds are very useful fluorinating agents. ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235U.
Group 18 Elements
Group 18 consists of elements: helium, neon, argon, krypton, xenon, radon and oganesson. All these are gases and chemically unreactive. They form very few compounds, because of this they are termed as noble gases.
Occurrence: All these gases except radon and oganesson occur in the atmosphere. Their atmospheric abundance in dry air is ~ 1% by volume of which argon is the major constituent.
Electronic Configuration: All noble gases have general electronic configuration ns2np6 except helium which has 1s2. Many of the properties of noble gases including their inactive nature are ascribed to their closed shell structures.
Ionisation Enthalpy: Due to stable electronic configuration these gases exhibit very high ionisation enthalpy. However, it decreases down the group with increase in atomic size.
Atomic Radii: Atomic radii increase down the group with increase in atomic number.
Electron Gain Enthalpy: Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have large positive values of electron gain enthalpy.
Physical Properties: All the noble gases are monoatomic. They are colourless, odourless and tasteless. They are sparingly soluble in water. They have very low melting and boiling points because the only type of interatomic interaction in these elements is weak dispersion forces. Helium has the lowest boiling point (4.2 K) of any known substance. It has an unusual property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics.
Chemical Properties: In general, noble gases are least reactive. Their inertness to chemical reactivity is attributed to the following reasons:
- The noble gases except helium (1s2) have completely filled ns2np6 electronic configuration in their valence shell.
- (ii) They have high ionisation enthalpy and more positive electron gain enthalpy.
Uses:
- Helium is a non-inflammable and light gas. Hence, it is used in filling balloons for meteorological observations. It is also used in gas-cooled nuclear reactors. Liquid helium (b.p. 4.2 K) finds use as cryogenic agent for carrying out various experiments at low temperatures. It is used to produce and sustain powerful superconducting magnets which form an essential part of modern NMR spectrometers and Magnetic Resonance Imaging (MRI) systems for clinical diagnosis. It is used as a diluent for oxygen in modern diving apparatus because of its very low solubility in blood.
- Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes. Neon bulbs are used in botanical gardens and in green houses.
- Argon is used mainly to provide an inert atmosphere in high temperature metallurgical processes (arc welding of metals or alloys) and for filling electric bulbs. It is also used in the laboratory for handling substances that are air-sensitive. There are no significant uses of Xenon and Krypton. They are used in light bulbs designed for special purposes.
