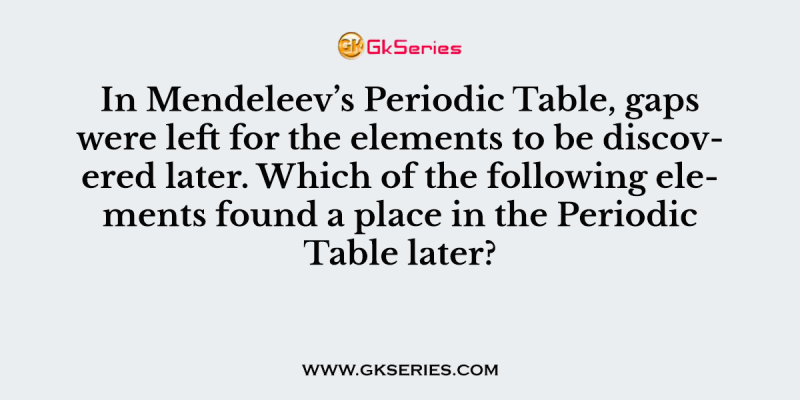
In Mendeleev’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the Periodic Table later?
READ MORE +
In Mendeleev’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the Periodic Table later?
READ MORE +
The properties of eka-aluminium predicted by Mendeleev are the same as the properties of later discovered element
READ MORE +
Where would you locate the element with electronic configuration 2, 8 in the Modern Periodic Table?
READ MORE +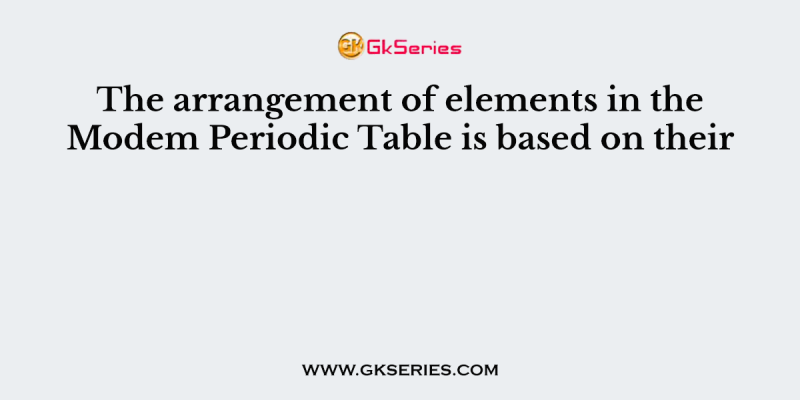
The arrangement of elements in the Modem Periodic Table is based on their
READ MORE +
At the time of Mendeleev, the number of elements known was
READ MORE +
Newlands relation is called
READ MORE +
Which one of the following statements is not correct about the trends in the properties of the elements of a group on going down in a group?
READ MORE +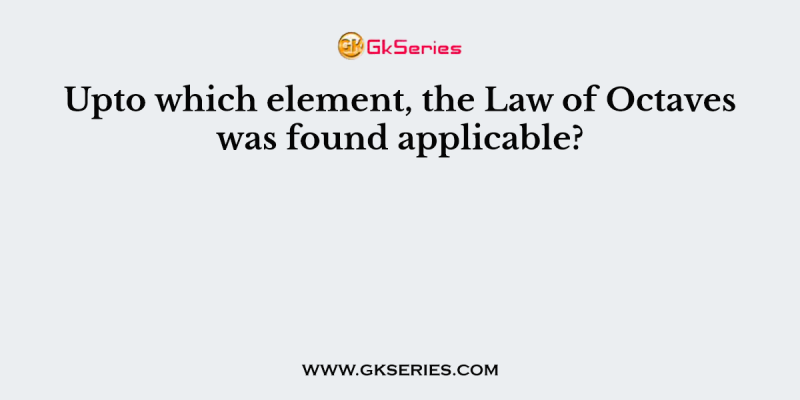
Upto which element, the Law of Octaves was found applicable?
READ MORE +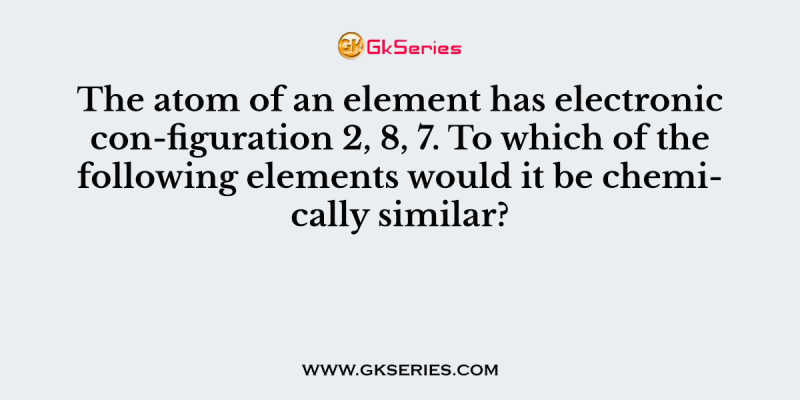
The atom of an element has electronic con-figuration 2, 8, 7. To which of the following elements would it be chemically similar?
READ MORE +
Magnesium has an atomic number of
READ MORE +
In period from left to right electron affinity of elements
READ MORE +
Recurrence of same pattern in periodic table is called its
READ MORE +