Answer:1:3
Answer:1:3
| Article and Schedule Quiz | Start Test! |
Answer:sum of the number of protons and neutrons is same but the number of protons is different.
Answer:Overall neutrality of atom.
Answer:The mass of electron is equal to the mass of neutron.
Answer:Characteristics of cathode rays depend upon the nature of gas present in the cathode ray tube.
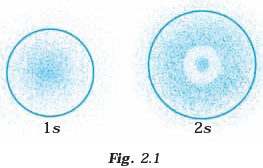
The density of dots in a region represents the probability density of finding electrons in the region.
On the basis of above diagram which of the following statements is incorrect?
Answer:The probability density of electrons for 2s orbital decreases uniformly as distance from the nucleus increases.
Answer:1s2 2s2 2p6 3s2 3p6 3d9 4s2
Answer:Electrons move in a circular path of fixed energy called orbits.
Answer:5, 0, 0, + 1/2
Answer:Amongst isoelectronic species, smaller the positive charge on the cation, smaller is the ionic radius
Answer:743 nm
Answer: – 30.6 eV
Answer:Li+2
(c = 3×108 ms–1 and NA = 6.02×1023 mol–1)
Answer:494 nm
Answer:K+, Cl–, Ca2+, Sc3+
Answer:12 and 5
| Missiles Mock Test | Start Test! |
| SSC MTS Mock Test | Start Test |
| IBPS CLERK MOCK TEST | Start Test |
| SSC MTS 2022 JULY 26 Shift 1 (ENGLISH) | Start Test! |
| SSC GD Previous Year Paper 2021 Nov 17 Shift - I (Hindi) | Start Test! |
| SSC CGL Tier - 1 PYP 2022 April 21 Shift- 1 (ENGLISH) | Start Test! |
| MPSC PAPER I MOCK TEST 1 (ENGLISH) | Start Test! |
| IB Security Assistant Mock test 1 (english) | Start Test! |
| UP POLICE CONSTABLE MOCK TEST 1 | Start Test! |
| DELHI POLICE CONSTABLE MOCK TEST 1 (HINDI) | Start Test! |