Answer:B > P > As > Bi
Answer:B > P > As > Bi
| Article and Schedule Quiz | Start Test! |
Answer:Strong attractive force between the atoms
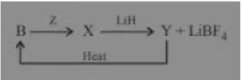
Which of the statement is true for the above sequence of reactions?
Answer:Z and Y are F2 and B2H6 respectively
Answer:Upper layer becomes violet
Answer:The oxygen-oxygen bond length in ozone is identical with that of molecular oxygen
Answer:2, 4
Answer:C−Cl > C−Br > C−I
Answer:By action of ultraviolet rays on oxygen molecule
Answer: Copper (I) metaborate is colourless
Answer:Reaction between Zn and conc. H2SO4
Answer:It has a polymeric structure
| Missiles Mock Test | Start Test! |
| SSC MTS Mock Test | Start Test |
| IBPS CLERK MOCK TEST | Start Test |
| SSC MTS 2022 JULY 26 Shift 1 (ENGLISH) | Start Test! |
| SSC GD Previous Year Paper 2021 Nov 17 Shift - I (Hindi) | Start Test! |
| SSC CGL Tier - 1 PYP 2022 April 21 Shift- 1 (ENGLISH) | Start Test! |
| MPSC PAPER I MOCK TEST 1 (ENGLISH) | Start Test! |
| IB Security Assistant Mock test 1 (english) | Start Test! |
| UP POLICE CONSTABLE MOCK TEST 1 | Start Test! |
| DELHI POLICE CONSTABLE MOCK TEST 1 (HINDI) | Start Test! |