Answer:37.56
Answer:37.56
Answer:KNO3 (∆H = 35.4 kJ mol-1)
| Article and Schedule Quiz | Start Test! |
Answer:10 kJ
Answer:For a cyclic process, q = -w
Answer:-100 × kJ/mol
Answer:-110.5 kJ
Answer:Gibbs Free Energy
Answer:Sublimation of Naphthalene
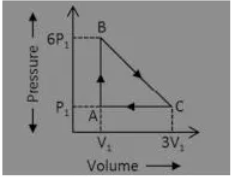
Answer:5P1V1
Answer:10 kJ
Answer:-47 kJ
Answer:1.11 × 104 kcal
Answer:∆S = 0
Answer:350 kJ
Answer:Cl2(g)
Answer: 84 J mol-1K-1
Answer:Absolute Entropy
| Missiles Mock Test | Start Test! |
| SSC MTS Mock Test | Start Test |
| IBPS CLERK MOCK TEST | Start Test |
| SSC MTS 2022 JULY 26 Shift 1 (ENGLISH) | Start Test! |
| SSC GD Previous Year Paper 2021 Nov 17 Shift - I (Hindi) | Start Test! |
| SSC CGL Tier - 1 PYP 2022 April 21 Shift- 1 (ENGLISH) | Start Test! |
| MPSC PAPER I MOCK TEST 1 (ENGLISH) | Start Test! |
| IB Security Assistant Mock test 1 (english) | Start Test! |
| UP POLICE CONSTABLE MOCK TEST 1 | Start Test! |
| DELHI POLICE CONSTABLE MOCK TEST 1 (HINDI) | Start Test! |