Answer:Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
Answer:Its tendency to gain a single electron in its valence shell to attain stable electronic configuration.
Answer:Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions. Due to smal size it cannot exist free.
| Article and Schedule Quiz | Start Test! |
Answer:LiH < NaH < KH < RbH < CsH
Answer:Tritium
(A) H2O2 + 2HI → I2 + 2H2O (B) HOCl + H2O2 → H3O+ + Cl+ O2
Which of the following statements is correct about H2O2 with reference to these reactions? Hydrogen perioxide is ________.
Answer:an oxidising agent in (A) and reducing agent in (B)
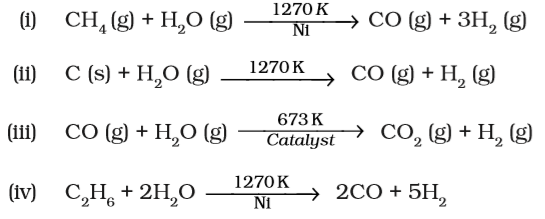
Answer:(iii)
Answer:sodium sulphate and hydrogen peroxide
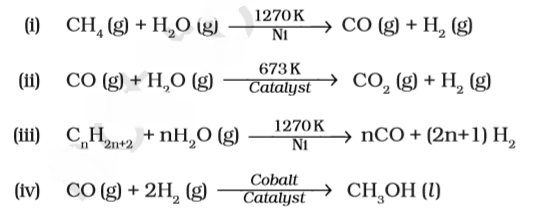
Answer:(iv)
Answer:Groups 7, 8, 9
Answer:A, B
Answer:A, C
Answer:C, D
| Missiles Mock Test | Start Test! |
| SSC MTS Mock Test | Start Test |
| IBPS CLERK MOCK TEST | Start Test |
| SSC MTS 2022 JULY 26 Shift 1 (ENGLISH) | Start Test! |
| SSC GD Previous Year Paper 2021 Nov 17 Shift - I (Hindi) | Start Test! |
| SSC CGL Tier - 1 PYP 2022 April 21 Shift- 1 (ENGLISH) | Start Test! |
| MPSC PAPER I MOCK TEST 1 (ENGLISH) | Start Test! |
| IB Security Assistant Mock test 1 (english) | Start Test! |
| UP POLICE CONSTABLE MOCK TEST 1 | Start Test! |
| DELHI POLICE CONSTABLE MOCK TEST 1 (HINDI) | Start Test! |