Surface Chemistry is a branch of chemistry that deals with the properties of surfaces or phase boundaries and with the chemical changes occurring at a surface or interface.
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent.
Adsorption in action
(i) If a gas like O2, H2, CO, Cl2, NH3 or SO2 is taken in a closed vessel containing powdered charcoal, it is observed that the pressure of the gas in the enclosed vessel decreases. The gas molecules concentrate at the surface of the charcoal, i.e., gases are adsorbed at the surface.
(ii) In a solution of an organic dye, say methylene blue, when animal charcoal is added and the solution is well shaken, it is observed that the filtrate turns colourless. The molecules of the dye, thus, accumulate on the surface of charcoal, i.e., are adsorbed.
(iii) Aqueous solution of raw sugar, when passed over beds of animal charcoal, becomes colourless as the colouring substances are adsorbed by the charcoal.
(iv) The air becomes dry in the presence of silica gel because the water molecules get adsorbed on the surface of the gel.
Distinction between Adsorption and Absorption
The main difference between absorption and adsorption is that in adsorption, the substance is concentrated only at the surface and does not penetrate through the surface to the bulk of the adsorbent, while in absorption, the substance is uniformly distributed throughout the bulk of the solid.
Both adsorption and absorption can take place simultaneously also. The term sorption is used to describe both the processes.
Mechanism of Adsorption
Adsorption arises due to the fact that the surface particles of the adsorbent are not in the same environment as the particles inside the bulk. Inside the adsorbent all the forces acting between the particles are mutually balanced but on the surface the particles are not surrounded by atoms or molecules of their kind on all sides, and hence they possess unbalanced or residual attractive forces.
These forces of the adsorbent are responsible for attracting the adsorbate particles on its surface. The extent of adsorption increases with the increase of surface area per unit mass of the adsorbent at a given temperature and pressure.
Types of Adsorption
There are mainly two types of adsorption of gases on solids. If accumulation of gas on the surface of a solid occurs on account of weak van der Waals’ forces, the adsorption is termed as physical adsorption or physisorption.
When the gas molecules or atoms are held to the solid surface by chemical bonds, the adsorption is termed chemical adsorption or chemisorption.
The chemical bonds may be covalent or ionic in nature. Chemisorption involves a high energy of activation and is, therefore, often referred to as activated adsorption.
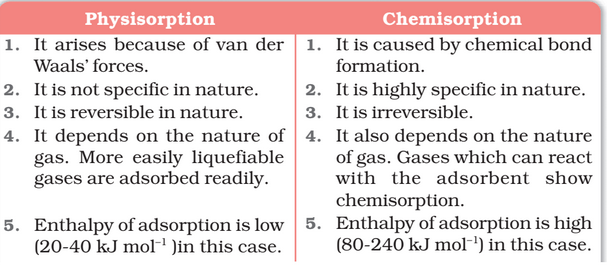
Comparison of Physisorption and Chemisorption
Adsorption Isotherms
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption isotherm.
Freundlich adsorption isotherm: Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
Adsorption from Solution Phase
Solids can adsorb solutes from solutions also. When a solution of acetic acid in water is shaken with charcoal, a part of the acid is adsorbed by the charcoal and the concentration of the acid decreases in the solution. Similarly, the litmus solution when shaken with charcoal becomes colourless.
The following observations have been made in the case of adsorption from solution phase:
(i) The extent of adsorption decreases with an increase in temperature.
(ii) The extent of adsorption increases with an increase of surface area of the adsorbent.
(iii) The extent of adsorption depends on the concentration of the solute in solution.
(iv) The extent of adsorption depends on the nature of the adsorbent and the adsorbate.
Applications of Adsorption
The phenomenon of adsorption finds a number of applications. Important ones are listed here:
(i) Production of high vacuum: The remaining traces of air can be adsorbed by charcoal from a vessel evacuated by a vacuum pump to give a very high vacuum.
(ii) Gas masks: Gas mask (a device which consists of activated charcoal or mixture of adsorbents) is usually used for breathing in coal mines to adsorb poisonous gases.
(iii) Control of humidity: Silica and aluminium gels are used as adsorbents for removing moisture and controlling humidity.
(iv) Removal of colouring matter from solutions: Animal charcoal removes colours of solutions by adsorbing coloured impurities.
(v) Heterogeneous catalysis: Adsorption of reactants on the solid surface of the catalysts increases the rate of reaction. There are many gaseous reactions of industrial importance involving solid catalysts. Manufacture of ammonia using iron as a catalyst, manufacture of H2SO4 by contact process and use of finely divided nickel in the hydrogenation of oils are excellent examples of heterogeneous catalysis.
(vi) Separation of inert gases: Due to the difference in degree of adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
(vii) In curing diseases: A number of drugs are used to kill germs by getting adsorbed on them.
(viii) Froth floatation process: A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method using pine oil and frothing agent.
(ix) Adsorption indicators: Surfaces of certain precipitates such as silver halides have the property of adsorbing some dyes like eosin, fluorescein, etc. and thereby producing a characteristic colour at the end point.
(x) Chromatographic analysis: Chromatographic analysis based on the phenomenon of adsorption finds a number of applications in analytical and industrial fields.
Catalysis
A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process.
Catalysts and their associated catalytic reactions come in three main types: homogeneous catalysts, heterogeneous catalysts and biocatalysts (usually called enzymes). Less common but still important types of catalyst activities include photocatalysis, environmental catalysis and green catalytic processes.
Homogeneous and Heterogeneous Catalysis
Heterogeneous catalysis: This involves the use of a catalyst in a different phase from the reactants. Typical examples involve a solid catalyst with the reactants as either liquids or gases.
Homogeneous catalysis: This has the catalyst in the same phase as the reactants. Typically everything will be present as a gas or contained in a single liquid phase. The examples contain one of each of these.
Adsorption Theory of Heterogeneous Catalysis
Modern Adsorption theory of heterogeneous catalysis is the mixture of moderate compound hypothesis and the old adsorption hypothesis or old adsorption theory. Old adsorption theory lacked specificity so there was a need for modern adsorption theory.
According to adsorption theory of heterogeneous catalyst, there are free valencies in the catalyst on which reactant molecules get attached. The mechanism of adsorption theory of heterogeneous catalysis involves following steps –
- Diffusion of Reactant Molecules: In this step reactant molecules get diffused towards the surface of the catalyst.
- Adsorption: In this step reactant molecules get adsorbed on the surface of the solid catalyst or form loose bonds with the free valencies of the catalyst.
- Intermediate Complex Formation: In this step adsorbed reactant molecules on the surface of the catalyst react with each other and form new stronger bonds with each other which leads to the formation of an intermediate.
- Desorption: In this step intermediate converts into product as it loses its affinity towards the catalyst. The product molecule gets desorbed from the surface of the catalyst.
- Diffusion of Product Molecules: In this step desorbed product molecules from the surface of the catalyst get diffused away from the catalyst.
Shape-Selective Catalysis by Zeolites
A catalytic reaction which depends upon the pore structure of the catalyst and on the size of the reactant and the product molecules is called shape-selective catalysis. For example, catalysis by zeolites is a shape-selective catalysis. The pore size present in the zeolites ranges from 260-740 pm. Thus, molecules having a pore size more than this cannot enter the zeolite and undergo the reaction. ZSM (5) is zeolite sieve of porosity 5. It is used to convert alcohols into hydrocarbons.
Enzyme Catalysis
Enzyme catalysis is the increase in the rate of a process by a biological molecule, an "enzyme". Most enzymes are proteins, and most such processes are chemical reactions. Within the enzyme, generally catalysis occurs at a localized site, called the active site.
Characteristics of enzyme catalysis
Enzyme catalysis is unique in its efficiency and high degree of specificity. The following characteristics are exhibited by enzyme catalysts:
- Most highly efficient: One molecule of an enzyme may transform one million molecules of the reactant per minute.
- Highly specific nature: Each enzyme is specific for a given reaction, i.e., one catalyst cannot catalyse more than one reaction. For example, the enzyme urease catalyses the hydrolysis of urea only. It does not catalyse hydrolysis of any other amide.
- Highly active under optimum temperature: The rate of an enzyme reaction becomes maximum at a definite temperature, called the optimum temperature. On either side of the optimum temperature, the enzyme activity decreases. The optimum temperature range for enzymatic activity is 298-310K. Human body temperature being 310 K is suited for enzyme-catalysed reactions.
- Highly active under optimum pH: The rate of an enzyme-catalysed reaction is maximum at a particular pH called optimum pH, which is between pH values 5-7.
- Increasing activity in presence of activators and co-enzymes: The enzymatic activity is increased in the presence of certain substances, known as co-enzymes. It has been observed that when a small non-protein (vitamin) is present along with an enzyme, the catalytic activity is enhanced considerably.
- Influence of inhibitors and poisons: Like ordinary catalysts, enzymes are also inhibited or poisoned by the presence of certain substances. The inhibitors or poisons interact with the active functional groups on the enzyme surface and often reduce or completely destroy the catalytic activity of the enzymes. The use of many drugs is related to their action as enzyme inhibitors in the body.
Colloids
A colloid is a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium.
Classification of Colloids
Colloids are classified on the basis of the following criteria:
(i) Physical state of dispersed phase and dispersion medium
(ii) Nature of interaction between dispersed phase and dispersion medium
(iii) Type of particles of the dispersed phase.
Preparation of Colloids
A few important methods for the preparation of colloids are as follows:
- Chemical methods: Colloidal dispersions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to formation of sols.
- Electrical disintegration or Bredig’s Arc method: This process involves dispersion as well as condensation. Colloidal sols of metals such as gold, silver, platinum, etc., can be prepared by this method.
- Peptization: Peptization may be defined as the process of converting a precipitate into colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte. The electrolyte used for this purpose is called peptizing agent. This method is applied, generally, to convert a freshly prepared precipitate into a colloidal sol.
Purification of Colloidal Solutions
The purification of colloidal solution is carried out by the following methods:
(i) Dialysis: It is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane.
(ii) Electro-dialysis: Ordinarily, the process of dialysis is quite slow. It can be made faster by applying an electric field if the dissolved substance in the impure colloidal solution is only an electrolyte.
(iii) Ultrafiltration: Ultrafiltration is the process of separating the colloidal particles from the solvent and soluble solutes present in the colloidal solution by specially prepared filters, which are permeable to all substances except the colloidal particles.
Properties of Colloidal Solutions
Various properties exhibited by the colloidal solutions are described below:
(i) Colligative properties: Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution.
Tyndall effect: If a homogeneous solution placed in dark is observed in the direction of light, it appears clear and, if it is observed from a direction at right angles to the direction of light beam, it appears perfectly dark.
(iii) Colour: The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles. The wavelength of light further depends on the size and nature of the particles.
(iv) Brownian movement: When colloidal solutions are viewed under a powerful ultramicroscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view.
(v) Charge on colloidal particles: Colloidal particles always carry an electric charge. The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative.
Emulsions
Emulsion is a type of colloid formed by combining two liquids that normally don't mix. In an emulsion, one liquid contains a dispersion of the other liquid. Common examples of emulsions include egg yolk, butter, and mayonnaise. The process of mixing liquids to form an emulsion is called emulsification.
There are two types of emulsions.
(i) Oil dispersed in water (O/W type) and
(ii) Water dispersed in oil (W/O type).
Colloids around us
Most of the substances, we come across in our daily life, are colloids. The meals we eat, the clothes we wear, the wooden furniture we use, the houses we live in, the newspapers we read, are largely composed of colloids.
Following are the interesting and noteworthy examples of colloids:
(i) Blue colour of the sky: Dust particles along with water suspended in air scatter blue light which reaches our eyes and the sky looks blue to us.
(ii) Fog, mist and rain: When a large mass of air containing dust particles, is cooled below its dewpoint, the moisture from the air condenses on the surfaces of these particles forming fine droplets.
(iii) Food articles: Milk, butter, halwa, ice creams, fruit juices, etc., are all colloids in one form or the other.
(iv) Blood: It is a colloidal solution of an albuminoid substance. The styptic action of alum and ferric chloride solution is due to coagulation of blood forming a clot which stops further bleeding.
(v) Soils: Fertile soils are colloidal in nature in which humus acts as a protective colloid. On account of colloidal nature, soils adsorb moisture and nourishing materials.
(vi) Formation of delta: River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in its deposition with the formation of delta.
