Minerals are solid substances that are present in nature and can be made of one element or more elements combined together (chemical compounds). Gold, Silver and carbon are elements that form minerals on their own. They are called native elements.
A metal is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically malleable or ductile.
Occurrence of Metals
Mostly, the metals occur in nature in a combined state but sometimes they can also occur in the free state.
A native metal is a metal found in its metallic state naturally, either in pure form or in the form of an alloy. Most metals can't resist natural processes like oxidation, corrosion, etc.
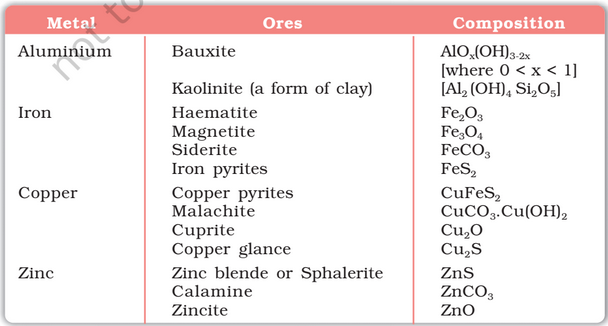
Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals that can be mined, treated and sold at a profit. Ore is extracted from the earth through mining and treated or refined, often via smelting, to extract the valuable metals or minerals.
The entire scientific and technological process used for isolation of the metal from its ore is known as metallurgy.
An ore rarely contains only a desired substance. It is usually contaminated with earthly or undesired materials known as gangue.
The extraction and isolation of metals from ores involves the following major steps:
- Concentration of the ore
- Isolation of the metal from its concentrated ore and
- Purification of the metal
Concentration of Ores
Removal of the unwanted materials (e.g., sand, clays, etc.) from the ore is known as concentration, dressing or benefaction.
Some of the important procedures for concentration of ore are described below.
Hydraulic Washing: This is based on the difference between specific gravities of the ore and the gangue particles. It is therefore a type of gravity separation.
Magnetic Separation: This is based on differences in magnetic properties of the ore components.
Froth Floatation Method: This method is used for removing gangue from sulphide ores. In this process, a suspension of the powdered ore is made with water.
Leaching: Leaching is often used if the ore is soluble in some suitable solvent. Following examples illustrate the procedure:
- Leaching of alumina from bauxite: Bauxite is the principal ore of aluminium. It usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities.
- Other examples: In the metallurgy of silver and gold, the respective metal is leached with a dilute solution of NaCN or KCN in the presence of air, which supplies O2.
Extraction of Crude Metal from Concentrated Ore
To extract metal from concentrated ore, it must be converted to a form which is suitable for reduction to metal. Usually sulphide ores are converted to oxide before reduction because oxides are easier to reduce. Thus isolation of metals from concentrated ore involves two major steps viz.,
(a) Conversion to oxide:
- Calcination: Calcinaton involves heating. It removes the volatile matter which escapes leaving behind the metal oxide:
- Roasting: In roasting, the ore is heated in a regular supply of air in a furnace at a temperature below the melting point of the metal.
(b) Reduction of the oxide to metal: Reduction of the metal oxide usually involves heating it with a reducing agent, for example C, or CO or even another metal.
Thermodynamic Principles of Metallurgy
Some basic concepts of thermodynamics help us in understanding the theory of metallurgical transformations. Gibbs energy is the most significant term.
The change in Gibbs energy, ∆G for any process at any specified temperature, is described by the equation: ∆G = ∆H – T∆S
Applications of Thermodynamic Principles of Metallurgy
- Extraction of iron from its oxides: After concentration, mixture of oxide ores of iron (Fe2O3, Fe3O4) is subjected to calcination/roasting to remove water, to decompose carbonates and to oxidise sulphides.
- Extraction of copper from cuprous oxide: In the graph of ∆rG0 vs T for the formation of oxides the Cu2O line is almost at the top. So it is quite easy to reduce oxide ores of copper directly to the metal by heating with coke.
- Extraction of zinc from zinc oxide: The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in the case of copper.
Electrochemical Principles Metallurgy
The principles of metallurgy are effective in the reduction of metal ions to their respective metals in their solution (or) molten states. The reduction is carried out through electrolysis (or) using reducing elements. Such methods are based on electrochemical principles.
![]()
These electrochemical principles are explained in
ΔG° = -nE°F
n = Number of Electrons Gained
E° = Electrode Potential of Redox Couple
The value of E° for a metal depends on its reactivity. Thus, it differs from metal to metal. More reactive metals have high E°, whereas less reactive metals have low E°.
Oxidation Reduction
Besides reductions, some extractions are based on oxidation particularly for non-metals. A very common example of extraction based on oxidation is the extraction of chlorine from brine (chlorine is abundant in sea water as common salt).
![]()
Refining
A metal extracted by any method is usually contaminated with some impurity. For obtaining metals of high purity, several techniques are used depending upon the differences in properties of the metal and the impurity. Some of them are listed below.
(a) Distillation: This is very useful for low boiling metals like zinc and mercury. The impure metal is evaporated to obtain the pure metal as distillate.
(b) Liquation: In this method a low melting metal like tin can be made to flow on a sloping surface. In this way it is separated from higher melting impurities.
(c) Electrolytic refining: In this method, the impure metal is made to act as anode. A strip of the same metal in pure form is used as cathode.
(d) Zone refining: This method is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
(e) Vapour phase refining: In this method, the metal is converted into its volatile compound which is collected and decomposed to give pure metal. So, the two requirements are:
- The metal should form a volatile compound with an available reagent,
- The volatile compound should be easily decomposable, so that the recovery is easy.
Uses of Uses of Aluminium, Copper, Zinc and Iron
Aluminium: Aluminium foils are used as wrappers for food materials. The fine dust of the metal is used in paints and lacquers. Aluminium, being highly reactive, is also used in the extraction of chromium and manganese from their oxides. Wires of aluminium are used as electricity conductors. Alloys containing aluminium, being light, are very useful.
Copper: Copper is used for making wires used in electrical industry and for water and steam pipes. It is also used in several alloys that are rather tougher than the metal itself, e.g., brass (with zinc), bronze (with tin) and coinage alloy (with nickel).
Zinc: Zinc is used for galvanising iron. It is also used in large quantities in batteries. It is constituent of many alloys, e.g., brass, (Cu 60%, Zn 40%) and german silver (Cu 25-30%, Zn 25-30%, Ni 40–50%). Zinc dust is used as a reducing agent in the manufacture of dye-stuffs, paints, etc.
Iron: Cast iron, which is the most important form of iron, is used for casting stoves, railway sleepers, gutter pipes, toys, etc. It is used in the manufacture of wrought iron and steel. Wrought iron is used in making anchors, wires, bolts, chains and agricultural implements. Steel finds a number of uses. Alloy steel is obtained when other metals are added to it.
