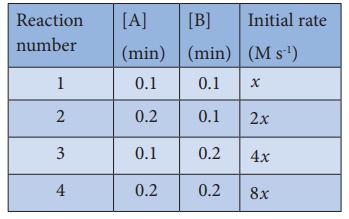
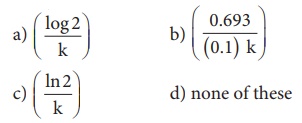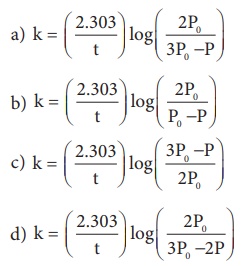This reaction follows first order kinetics. The rate constant at particular temperature is 2.303 × 10−2 hour−1 . The initial concentration of cyclopropane is 0.25M. What will be the concentration of cyclopropane after 1806 minutes? (log 2 = 0.3010)
This reaction follows first order kinetics. The rate constant at particular temperature is 2.303 × 10−2 hour−1 . The initial concentration of cyclopropane is 0.25M. What will be the concentration of cyclopropane after 1806 minutes? (log 2 = 0.3010)
Answer: 0.215M