Answer: (1x10-2) e-60x
Answer: (1x10-2) e-60x
Answer: 20 min
| Article and Schedule Quiz | Start Test! |
Answer: Without knowing the rate constant, t1/2 cannot be determined from the given data
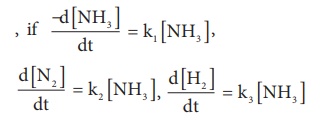
then the relation between k1, k2 and k3 is
Answer: 1.5 k1 = 3 k2 = k3
Answer: rate is independent of the surface coverage
Answer: (mol-1/2 L1/2s-1), (mol L-1 s-1)
Answer: Activation energy
Consider the following statements :
(i) increase in concentration of the reactant increases the rate of a zero order reaction.
(ii) rate constant k is equal to collision frequency A if Ea = 0
(iii) rate constant k is equal to collision frequency A if Ea = °
(iv) a plot of ln(k) vs T is a straight line
(v) a plot of ln (k) vs 1/T is a straight line with a positive slope.
Correct statements are
Answer: (ii) only
In a reversible reaction, the enthalpy change and the activation energy in the forward direction are respectively −x kJ mol−1 and y kJ mol−1 . Therefore , the energy of activation in the backward direction is
a) b) c) d)Answer: ( x + y ) × 103 J mol−1
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 200K to 400K? (R = 8.314 JK-1mol-1)
Answer:434.65 J mol−1K−1
| Missiles Mock Test | Start Test! |
| SSC MTS Mock Test | Start Test |
| IBPS CLERK MOCK TEST | Start Test |
| SSC MTS 2022 JULY 26 Shift 1 (ENGLISH) | Start Test! |
| SSC GD Previous Year Paper 2021 Nov 17 Shift - I (Hindi) | Start Test! |
| SSC CGL Tier - 1 PYP 2022 April 21 Shift- 1 (ENGLISH) | Start Test! |
| MPSC PAPER I MOCK TEST 1 (ENGLISH) | Start Test! |
| IB Security Assistant Mock test 1 (english) | Start Test! |
| UP POLICE CONSTABLE MOCK TEST 1 | Start Test! |
| DELHI POLICE CONSTABLE MOCK TEST 1 (HINDI) | Start Test! |