Chemical kinetics is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with thermodynamics, which deals with the direction in which a process occurs but in itself tells nothing about its rate.
Rate of a Chemical Reaction
The rate at which the concentration of reactant or product participating in a chemical reaction alters is called rate of reaction.
Rate of reaction = change in concentration/ time = (mol/litre)/time
Reactant (R) --> Product.
Rate [R]
Rate = k[R]
k = rate constant or velocity constant.
Let one mole of the reactant A produce one mole of the product B.
Let at time t1
[A]1 and [B]1 = Concentrations of A and B
Let at time t2
[R]2 and [P]2 = Concentrations of A and B
Rate of disappearance of A = Decrease in concentration of R / Time taken = -∆[A]/∆t
Rate of appearance of B = Increase in concentration of P / Time taken = +∆[B]/∆t
When two or more reactants combine with each other the molecules of the respective reactants collide with each other to form the product. The collision between the molecules increases with the increase in concentration of the reactants and thereby increases the rate of reaction.
Factors Influencing Rate of a Reaction
There are four main factors that can affect the reaction rate of a chemical reaction:
- Reactant concentration: Increasing the concentration of one or more reactants will often increase the rate of reaction. This occurs because a higher concentration of a reactant will lead to more collisions of that reactant in a specific time period.
- Physical state of the reactants and surface area: If reactant molecules exist in different phases, as in a heterogeneous mixture, the rate of reaction will be limited by the surface area of the phases that are in contact.
- Temperature: An increase in temperature typically increases the rate of reaction. An increase in temperature will raise the average kinetic energy of the reactant molecules.
- Presence of a catalyst: A catalyst is a substance that accelerates a reaction by participating in it without being consumed. Catalysts provide an alternate reaction pathway to obtain products. They are critical to many biochemical reactions.
Dependence of Rate on Concentration
The dependence of the rate on concentration is summarized in a rate law. The temperature at which the reaction occurs - Most reactions also proceed faster when the temperature is raised. The presence of a catalyst - Most biochemical reactions will not occur without the aid of proteins which act as enzymes.
Rate Expression and Rate Constant
The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants participating in it.
Expression
For a reaction given by:
aA + bB → cC + dD
Where a, b, c, and d are the stoichiometric coefficients of the reactants or products, the rate equation for the reaction is given by:
Rate ∝ [A]x[B]y ⇒ Rate = k[A]x[B]y
Where,
[A] & [B] denote the concentrations of the reactants A and B.
x & y denote the partial reaction orders for reactants A & B (which may or may not be equal to their stoichiometric coefficients a & b).
The proportionality constant ‘k’ is the rate constant of the reaction.
Order of a Reaction
The order of reaction can be defined as the power dependence of rate on the concentration of all reactants. For example, the rate of a first-order reaction is dependent solely on the concentration of one species in the reaction. Some characteristics of the reaction order for a chemical reaction are listed below.
- Reaction order represents the number of species whose concentration directly affects the rate of reaction.
- It can be obtained by adding all the exponents of the concentration terms in the rate expression.
- The order of reaction does not depend on the stoichiometric coefficients corresponding to each species in the balanced reaction.
- The reaction order of a chemical reaction is always defined with the help of reactant concentrations and not with product concentrations.
- The value of the order of reaction can be in the form of an integer or a fraction. It can even have a value of zero.
Molecularity of a Reaction
The molecularity of a reaction is defined as the number of reacting molecules which collide simultaneously to bring about a chemical reaction. In other words, the molecularity of an elementary reaction is defined as the number of reactant molecules taking part in the reaction.
Integrated Rate Equations
The rate law is a differential equation, meaning that it describes the change in concentration of reactant(s) per change in time. Using calculus, the rate law can be integrated to obtain an integrated rate equation that links concentrations of reactants or products with time directly. The integrated rate equations are different for the reactions of different reaction orders.
Zero Order Reactions
Zero order reaction simply means that the rate of reaction is independent of concentration of reactants. The Haber process produces ammonia from hydrogen and nitrogen gas. The reverse of this process (the decomposition of ammonia to form nitrogen and hydrogen) is a zero-order reaction.
First Order Reactions
A reaction that depends on the concentration of only one reactant (a unimolecular reaction). Other reactants can be present, but each will be zero-order. First-order reactions are very common. We have already encountered two examples of first-order reactions: the hydrolysis of aspirin and the reaction of t-butyl bromide with water to give t-butanol. Another reaction that exhibits apparent first-order kinetics is the hydrolysis of the anticancer drug cisplatin.
Half-Life of a Reaction
The half-life of a chemical reaction can be defined as the time taken for the concentration of a given reactant to reach 50% of its initial concentration (i.e. the time taken for the reactant concentration to reach half of its initial value). It is denoted by the symbol 't1/2' and is usually expressed in seconds.
Temperature Dependence of the Rate of a Reaction
Most of the chemical reactions are accelerated by increase in temperature.
For example, in decomposition of N2O5, the time taken for half of the original amount of material to decompose is 12 min at 50oC, 5 h at 25°C and 10 days at 0°C. You also know that in a mixture of potassium permanganate (KMnO4) and oxalic acid (H2C2O4), potassium permanganate gets decolourised faster at a higher temperature than that at a lower temperature.
It has been found that for a chemical reaction with rise in temperature by 10°, the rate constant is nearly doubled.
Effect of Catalyst
A catalyst is a substance which increases the rate of a reaction without itself undergoing any permanent chemical change. For example, MnO2 catalyses the following reaction so as to increase its rate considerably.
The Effect of Catalysts on the Activation Energy Barrier. Catalysts provide a new reaction pathway in which a lower A.E. is offered. A catalyst increases the rate of a reaction by lowering the activation energy so that more reactant molecules collide with enough energy to surmount the smaller energy barrier.
Collision Theory of Chemical Reactions
Collision theory states that the rate of a chemical reaction is proportional to the number of collisions between reactant molecules. The more often reactant molecules collide, the more often they react with one another, and the faster the reaction rate.
1. Chemical kinetics is the branch of chemistry which deals with the study of rates (or fastness) of chemical reactions, the factors affecting it and the mechanism by which the reactions proceed.
2. Rate of reaction is the change in concentration of reactants or products per unit time.
For a general reaction, A+B –> C
The rate of reaction
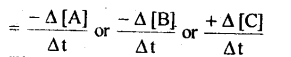
The negative sign indicates that the concentration is decreasing with time.
Unit for reaction rate is mol L-1s-1.
3. The rate of reaction is not a constant quantity (except for zero order reactions). It decreases as the reaction proceeds in the forward direction.
4. A rate law expresses a mathematical relationship between the reaction rate and the molar concentration of one or more reactants.
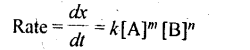
Where m and n are determined experimentally and represent the order of reaction with respect to A and B respectively, m + n represents the overall order of reaction.
5. Rate constant is the rate of reaction when the concentration of each of reacting species is unity. It is represented by ‘k’ It is also called specific reaction rate or velocity constant of reaction.
6. Order of reaction is defined as the sum of the exponents to which the concentration terms are raised in the rate equation (or rate law) of the reaction. It can be fraction, zero or any whole number.
7. Modularity of reaction is defined as the number of reacting particles (atoms or molecules or any other species), which collides simultaneously to bring about the chemical change.
It is a theoretical concept. Its value is always a whole number. It is never more than three. It cannot be zero.
8. First order reaction: A reaction is said to be first order if its reaction rate is determined by the variation of one concentration term only.
9. The integrated rate equation expresses the concentration of reactants as a function of time.
10. The integrated rate equation for a first order reaction is given as

11. Second order reaction: The reaction in which sum of powers of concentration terms in rate law equation is two.
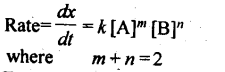
12. Zero order reaction: Those reactions in which rate of reaction does not change with concentration of the reactants.
Rate law for such a reaction is expressed as. Rate = k [A]°[B]°
13. Half life period: It is the time required for the initial concentration of the reactant to be reduced to half its value.
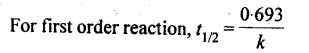
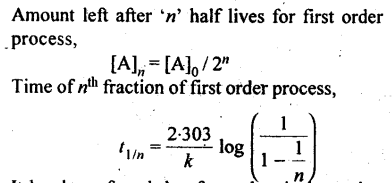
14. It has been found that for a chemical reaction with rise in temperature by 10 °C, the rate constant gets nearly doubled.
15. The temperature coefficient of a reaction is the ratio of the rate constants of the reaction at two temperatures differing from one another by 10°C. The two temperatures usually taken are 35 °C and 25 °C.
16. The variation of rate constants with temperature can be represented by the Arrhenius equation,
K=Ae-Ea/Rt
where A is a constant known as frequency factor, and Ea is-called the energy of activation.
From the above equation, the rate constants at two different temperatures are related as
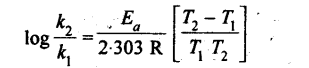
17. There are two important theories of reaction rates:
(i) Collision theory and,
(ii) Transition state theory.
