The nucleus is a collection of particles called protons, which are positively charged, and neutrons, which are electrically neutral. Protons and neutrons are in turn made up of particles called quarks. The chemical element of an atom is determined by the number of protons, or the atomic number, Z, of the nucleus.
Atomic Masses and Composition of Nucleus
The mass of an atom is very small, compared to a kilogram; for example, the mass of a carbon atom, 12C, is 1.992647 ×10 -26 kg. Kilogram is not a very convenient unit to measure such small quantities. Therefore, a different mass unit is used for expressing atomic masses. This unit is the atomic mass unit (u), defined as 1/12th of the mass of the carbon (12C) atom. According to this definition
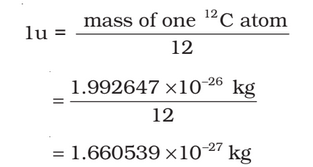
The atomic masses of various elements expressed in atomic mass unit (u) are close to being integral multiples of the mass of a hydrogen atom. There are, however, many striking exceptions to this rule. For example, the atomic mass of chlorine atom is 35.46 u.
Discovery of Neutron
In May 1932 James Chadwick announced that the core also contained a new uncharged particle, which he called the neutron. Chadwick was born in1891 in Manchester, England.
The composition of a nucleus can now be described using the following terms and symbols:
Z - atomic number = number of protons
N - neutron number = number of neutrons
A - mass number = Z + N = total number of protons and neutrons
One also uses the term nucleon for a proton or a neutron. Thus the number of nucleons in an atom is its mass number A.
Size of the Nucleus
It is found that nuclear radii range from 1-10 ´ 10-15 m. This radius is much smaller than that of the atom, which is typically 10-10 m. Thus, the nucleus occupies an extremely small volume inside the atom. The nuclei of some atoms are spherical, while others are stretched or flattened into deformed shapes.
Mass-Energy and Nuclear Binding Energy
Mass – Energy
Einstein showed from his theory of special relativity that it is necessary to treat mass as another form of energy. Before the advent of this theory of special relativity it was presumed that mass and energy were conserved separately in a reaction. However, Einstein showed that mass is another form of energy and one can convert mass-energy into other forms of energy, say kinetic energy and vice-versa. Einstein gave the famous mass-energy equivalence relation
E = mc2
Here the energy equivalent of mass m is related by the above equation and c is the velocity of light in vacuum and is approximately equal to 3×m s–1.
Nuclear binding energy
Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts: protons and neutrons, or, collectively, the nucleons. The binding energy of nuclei is always a positive number, since all nuclei require net energy to separate them into individual protons and neutrons.
Nuclear Force
The nuclear force is a force that acts between the protons and neutrons of atoms. The nuclear force is the force that binds the protons and neutrons in a nucleus together. This force can exist between protons and protons, neutrons and protons or neutrons and neutrons. This force is what holds the nucleus together.
Radioactivity
A. H. Becquerel discovered radioactivity in 1896 purely by accident. Radioactivity is the spontaneous emission of radiation in the form of particles or high energy photons resulting from a nuclear reaction. A substance that contains unstable atomic nuclei is considered to be radioactive. Radioactive decay is a random or stochastic process that occurs at the level of individual atoms.
Experiments performed subsequently showed that radioactivity was a nuclear phenomenon in which an unstable nucleus undergoes a decay. This is referred to as radioactive decay. Three types of radioactive decay occur in nature :
(i) α-decay in which a helium nucleus is emitted;
(ii) β-decay in which electrons or positrons (particles with the same mass as electrons, but with a charge exactly opposite to that of electron) are emitted;
(iii) γ-decay in which high energy (hundreds of keV or more) photons are emitted. Each of these decay will be considered in subsequent sub-sections.
Law of radioactive decay
In any radioactive sample, which undergoes α, β or γ-decay, it is found that the number of nuclei undergoing the decay per unit time is proportional to the total number of nuclei in the sample. If N is the number of nuclei in the sample and ∆N undergo decay in time ∆t then
![]()
or, ∆N/∆t = λN, where λ is called the radioactive decay constant or disintegration constant.
Alpha decay
Alpha decay or α-decay is a type of radioactive decay in which the atomic nucleus emits an alpha particle thereby transforming or decaying into a new atomic nucleus. Here the atomic mass number of the newly formed atom will be reduced by four and the atomic number will be reduced by two.
Beta decay
Beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide.
Gamma decay
Gamma decay is one type of radioactive decay that a nucleus can undergo. What separates this type of decay process from alpha or beta decay is that no particles are ejected from the nucleus when it undergoes this type of decay. Instead, a high energy form of electromagnetic radiation - a gamma ray photon - is released.
Nuclear Energy
Nuclear energy is the energy in the nucleus, or core, of an atom. Atoms are tiny units that make up all matter in the universe, and energy is what holds the nucleus together. There is a huge amount of energy in an atom's dense nucleus.
Fission
Nuclear fission is a process in nuclear physics in which the nucleus of an atom splits into two or more smaller nuclei as fission products, and usually some by-product particles. Hence, fission is a form of elemental transmutation.
Nuclear reactor
A nuclear reactor, formerly known as an atomic pile, is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion.
Nuclear fusion – energy generation in stars
Fusion powers stars and produces virtually all elements in a process called nucleosynthesis. The Sun is a main-sequence star, and, as such, generates its energy by nuclear fusion of hydrogen nuclei into helium.
When fusion is achieved by raising the temperature of the system so that particles have enough kinetic energy to overcome the coulomb repulsive behaviour, it is called thermonuclear fusion.
Thermonuclear fusion is the source of energy output in the interior of stars. The interior of the sun has a temperature of 1.5× K, which is considerably less than the estimated temperature required for fusion of particles of average energy. Clearly, fusion in the sun involves protons whose energies are much above the average energy.
Controlled thermonuclear fusion
Controlled thermonuclear fusion is potentially a nearly unlimited source of energy. In the United States, billions of dollars have been spent on more than 50 years of fusion research, primarily on two conventional approaches, magnetic confinement fusion and inertial confinement fusion.
